The Structural Protein Is Responsible for Healthy Joints and Skin
There’s a lot of talk these days about collagen — how we lose it as we age and how important it is to a healthy body and even our skin, as it’s found in various connective tissues.
This is the first of our Learning Center articles specifically on collagen, and why it’s important to you.
To start, here are some important things to know about the protein, which provides structure, strength, and support throughout the body:
⦁ Comprises one-third of the human body’s total protein.
⦁ The most abundant structural protein in the body.
⦁ The primary role is to maintain connective tissue and mechanical properties of the skin.
⦁ Vital for strength, regulation, and regeneration of tissue.
⦁ 28 different types of collagen, designated by Roman numerals (I to XXVIII).
Collagen has a high concentration of three specific amino acids: glycine, proline, and hydroxyproline.
Hydroxyproline is a metabolite of proline. Collagen has a characteristic triple-helix structure. Cross-linking between fibrils or strands creates strength and flexibility. Vitamin C plays an important role in this formation. A serious deficiency of vitamin C can result in scurvy, a disease that damages soft tissue maintenance and repair and can lead to issues like bleeding gums, loose teeth and bleeding under the skin.
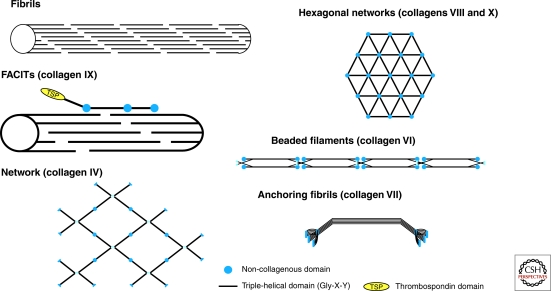
The arrangement of glycine and the other component amino acids are formed into fibrils. Certain types of collagen can form into knots, figure 8, or pretzel formations, depending on their type and function.
Collagen is an elastic protein with a resiliency (the ability to return to a resting state) of approximately 90 percent. The structural protein is subject to damage by Advanced Glycosylation End-Products (AGES), which are formed when glucose molecules are irreversibly attached to other proteins or molecules.
Protein function is directly related to shape. The attachment of an extra molecule changes the shape and inactivates the protein. The same is true for collagen; when glucose is attached, the collagen begins to lose elasticity and therefore loses its function. Glycosylation occurs continually but is dramatically increased when glucose or blood sugar levels are over 100. Loss of elasticity of collagen leads to fibrosis, scarring, sagging of skin, and the hampering of soft tissue repair.
Collagen is secreted by fibroblasts. These cells are common in the Extra-Cellular Matrix (ECM), which surrounds and supports cells. Fibroblasts are a major source of inflammatory cytokines in response to both acute injury and chronic inflammation. (JCI)
Collagens are deposited in the ECM but influence cellular activity through various cell receptors. The amounts and types of collagen vary between tissue types and the function required. Excess collagen creation by fibroblasts leads to fibrosis.
Different types of collagen serve to protect neurons, types that exhibit anti-angiogenesis (a process whereby cancer cells create their own abnormal blood supply), and direct anti-tumor effects. A derivative of one type of collagen has actually been approved as part of lung cancer treatment in China.
These roles are of course in addition to the supportive role collagen plays in muscle, bone, cartilage tendons, and skin.
Unfortunately, a variety of genetic conditions affect collagen and can lead to a multitude of diseases. (See the following table.)
In our next article, we will discuss the specific benefits of collagen supplementation — which is not meant to correct or treat any of the genetic conditions (listed as follows) resulting from mutations in collagen genes.
| Gene | Disease | References, databases |
| COL1A1 COL1A2 |
Osteogenesis imperfecta Ehlers–Danlos syndrome |
Marini et al. (2007) Dalgleish (1997, 1998) www.le.ac.uk/genetics/collagen Bodian and Klein (2009) http://collagen.stanford.edu/ |
| COL2A1 | Spondyloepiphyseal dysplasia Spondyloepimetaphyseal dysplasia, Achondrogenesis, hypochondrogenesis Kniest dysplasia, Stickler syndrome |
Bodian and Klein (2009) http://collagen.stanford.edu/ |
| COL3A1 | Ehlers–Danlos syndrome | Dalgleish (1997, 1998) www.le.ac.uk/genetics/collagen Bodian and Klein (2009) http://collagen.stanford.edu/ |
| COL4A1 | Familial porencephaly Hereditary angiopathy with nephropathy, aneurysms and muscle cramps syndrome |
Van Agtmael and Bruckner-Tuderman (2010) |
| COL4A3 COL4A4 | Alport syndrome Benign familial haematuria |
Van Agtmael and Bruckner-Tuderman (2010) |
| COL4A5 COL4A6 |
Alport syndrome Leiomyomatosis |
Bateman et al. (2009), Van Agtmael and Bruckner-Tuderman (2010) |
| COL5A1 COL5A2 |
Ehlers–Danlos syndrome | Callewaert et al. (2008) |
| COL6A1 COL6A2 COL6A3 |
Bethlem myopathy Ullrich congenital muscular dystrophy |
Lampe and Bushby (2005) |
| COL7A1 | Dystrophic epidermolysis bullosa | Fine (2010) |
| COL8A2 | Corneal endothelial dystrophies | Bateman et al. (2009) |
| COL9A1 COL9A2 |
Multiple epiphyseal dysplasia | Carter and Raggio (2009) |
| COL9A3 | Multiple epiphyseal dysplasia Autosomal recessive Stickler syndrome |
Carter and Raggio (2009) |
| COL10A1 | Schmid metaphyseal chondrodysplasia | Grant (2007) |
| COL11A1 | Stickler syndrome Marshall syndrome |
Carter and Raggio (2009) |
| COL11A2 | Stickler syndrome Marshall syndrome Otospondylomegaepiphyseal dysplasia Deafness |
Carter and Raggio (2009) |
| COL17A1 | Junctional epidermolysis bullosa-other | Has and Kern (2010) |
| COL18A1 | Knobloch syndrome | Nicolae and Olsen (2010) |
References:
Cold Spring Harb Perspect Biol. 2011 Jan; 3(1): a004978.
doi: 10.1101/cshperspect.a004978
PMCID: PMC3003457
PMID: 21421911
The Collagen Family
J Clin Invest. 2021 Oct 15;131(20):e149538.
doi: 10.1172/JCI149538.
Fibroblast pathology in inflammatory diseases:
Kevin Wei, Hung N Nguyen, Michael B Brenner
PMID: 34651581
PMCID: PMC8516469
DOI: 10.1172/JCI149538
Amino Acids. 2021 Oct;53(10):1493-1506.
doi: 10.1007/s00726-021-03072-x. Epub 2021 Sep 7.
The effects of collagen peptide supplementation on body composition, collagen synthesis, and recovery from joint injury and exercise: a systematic review:
Mishti Khatri 1, Robert J Naughton 1, Tom Clifford 2, Liam D Harper 3, Liam Corr 1
Affiliations expand
PMID: 34491424
PMCID: PMC8521576